BIO LAB CONCLUSION
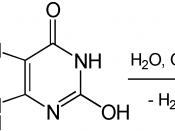
We did this experiment to explore how the concentration of peroxide effects the volume of produced Oxygen, presuming that as the concentration of celery gets higher, the volume of produced Oxygen becomes bigger. We made 5 trails for each of 6 different concentration-0.1%,1%,3%,5%, 10% and 15% and calculated the average data and standard deviation of each group to show the range of possible data range. According to the chemical equation H202âÂÂ02+H20, we uses 10 ml celery as the catalyst to increase the rate of experiment. Every time we add 20 ml peroxide as reactant to produce water and oxygen. To make sure that the concentration of peroxide is the only independent variable, the quantity of peroxide is always 20 ml and the celery is always 10ml.
By comparing the each group of data and the average between the concentrations of 0.1%, 1% and 3%, we found out that the volume of produced Oxygen goes bigger as the concentration of peroxide gets higher.
We assume that the volume is highest when the concentration is 15%, but the fact is that the volume is highest when the concentration is 5%. Also, by comparing the standard deviations, we found that the data from the groups of concentration with 5%, 10% and 15% are quit large which means the data may be not very accurate. In order to reduce these errors, we suggest doing another 5 trails of each of the 5%,10% and 15% one again to make sure the data are believable.
In general, the concentration of peroxide effect the volume of produced oxygen. As the concentration goes higher, the volume of Oxygen becomes bigger. Additionally, to make sure that the collected data is accurate enough to provide a conclusion, our experiment time should be at least 5...