Investigation to find out the Effect of Current on the Mass Deposited at the Cathode
The experiment I will be carrying out is aimed to observe the amount of Copper (Cu) metal deposited during the electrolysis of Copper Sulphate solution (CuSo4) using Copper electrodes, when certain variables of controlling the current, maybe with a computer, and also I could use a thermometer to show the change in temperature, so that it can be monitored. I also could have kept the size and separation of the electrodes the same. I also could have made sure that the crocodile clips were completely out of the electrolyte. Also I could have taken a much wider range of readings, from 0.2A to 1A at smaller intervals, and I could have also timed for different times. I could have also investigated the other variables, such as the temperature of the electrolyte, the concentration of the electrolyte, the separation of the electrodes, and the size of the electrodes
Equipment:
1. D.C. power supply - for providing the power for the experiment.
2. Ammeter - for measuring the amount of current flowing though the circuit.
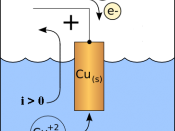
3. Electrodes [Anode (+) and Cathode (-)]
4. Variable Resistor - for changing the amount of current uses.
5. Circuit wires - for connecting up the apparatus to the power supply.
6. Beaker - for holding copper sulphate solution.
7. Copper sulphate solution - for doing the electrolysis experiment.
8. Distilled water and Acetone - for cleaning and drying the electrodes using them in descending order.
9. Stop clock - to measure the amount of time taken
There are roughly eight factors that will affect the outcome of the experiment.
1. Time
2. Current
3. Temperature
4. Molarity/Concentration of solution
5. Quantity of solution
6. Size of electrodes
7. Distance between the...


Well Done.
I didnt know much about this topic, but your piece is very insightful and provided a lot of information, well done!
0 out of 0 people found this comment useful.