The Important Role of Hydrogen Bonds
Hydrogen bonds are pretty much everywhere you go. You can't see them with the naked eye, but they have an important role in several biological structures that help sustain the life we know. In this paper I will define the hydrogen bond and explain the important role it has with the structure of DNA, the shape of proteins in the secondary and tertiary levels of protein structure, the unique properties of water and the cohesion-adhesion theory of transport in vascular plants.
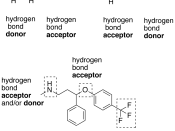
A hydrogen bond is one of three types of chemical bonds that occur between molecules. This bond comes as a result of dipole-dipole interaction; when one polar molecule with a partial positive charge is attracted to another polar molecule with a partial negative charge.
Hydrogen bonding occurs when a hydrogen donor, a hydrogen atom bonded to a strong electronegative atom (usually N, O or F), exists in the same area as a hydrogen acceptor, an electronegative atom with a lone pair of electrons (usually N, O or F, as well).
The hydrogen atom covalently bonded to an electronegative molecule produces the partial positive charge and the electronegative atom with a lone pair of electrons produces the partial negative charge.
This bond can either be intermolecular, between two like or unlike molecules, or intramolecular, within a single molecule, when the molecular geometry is favorable. These bonds tend to be stronger than ordinary dipole-dipole attractions but weaker than covalent and ionic bonds.
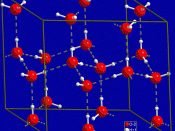
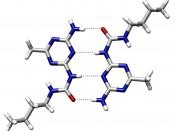
Hydrogen bonding is important to several biological processes to include the structure of DNA, the unique properties of water, the secondary and tertiary structures of protein, and the water transport system of plants.
Deoxyribonucleic acid, or DNA, is a macromolecule known as a nucleic acid. This molecule has been referred...