"SYNTHESIS of ASPIRIN"
PURPOSE:
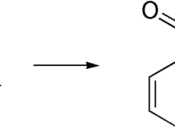
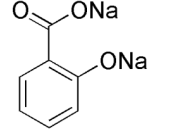
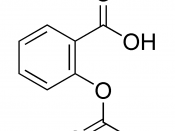
In this experiment is to obtain aspirin. Aspirin is prepared by acetylated salicylic acid I a process called esterification. Esterification is the reaction of the carboxyl (-COOH) group, and the -OH group to form a carboxyl ate ester group. In this case the source of the -OH group is the phenolic -OH attached to the ring of the salicylic acid. The acetyl group comes from acetic acid anhydride, and the reaction is catalyzed by phosphoric acid. Recrystalization is used in a mixed solvent of ethanol and water to purify the crude aspirin. Melting point determines the relative purity of obtained aspirin.
STRUCTURE EQUATION for the SYNTHESIS of ASPRIN:
PRODECURE:
Refer to the Mohrig Lab-book p. 41-49
RESULTS:
Amount of Methyl Salicylate = 1.008 g
Amount of Acetic anhydride = 2.0 ml
Mass of Aspirin = 0.425 g
Melting Point Range = 115- 122 0C
OBSERVATIONS:
% Yield of Aspirin = [actual yield/ theoretical yield] x 100
Mass of Obtained Aspirin = 0.425
g
% Yield of Aspirin = [0.425 / 1.008] x 100 = 42.16 %
Aspirin was obtained in a 42.16 % Yield from salicylic acid. Relatively pure white crystals of Aspirin were obtained. The purity was tested with melting range.
CHEMICALS USED:
ACETYLSALICYLIC ACID (ASPIRIN):
Molecular Formula: C9H8O4
Molecular Weight: 180.154
Melting Point: 135-136 0C
Boiling Point: 1400C
SALICYLIC ACID:
Molecular Formula: C7H6O3
Molecular Weight: 138.1226
Density: 1.44
Vapor Density: 4.8
Melting Point: 158- 162 0C
Boiling Point: 2110C at 20 mmHg
ACETIC ANHYDRIDE:
Molecular Formula: C4H6O3
Molecular Weight: 102.088
Density: 1.075 g/ml
Melting Point: -73.1 0C
Boiling Point: 139.50C
ACETIC ACID:
Molecular Formula: C2H4O2
Molecular Weight: 60.052
Density: 1.0497 g/ml
Melting Point:16.6 0C
Boiling Point: 117.9 0C
CONCLUSION:
Salicylic acid was converted into acetylsalicylic acid - Aspirin, which was obtained in 42.16 %...
Procedure?
Too bad you didn't include the experimental procedure. (Mohrig Lab-book?)
It would have interested me to read how aspirin is produced in a laboratory.
Other than that, a solid lab report.
2 out of 2 people found this comment useful.