CatalaseThe enzyme of catalase was first noticed 1811 by the French chemist Louis Jacques Thenard, after he reacted barium peroxide with nitric acid. He discovered hydrogen peroxide and suggested that it must have been broken down by a substance; it was not until 89 years later in 1900 that Oscar Loew named that substance. In 1937 James B.Summer crystallized catalase from beef liver and a year later he worked out its molecular weight. The amino acid sequence of bovine catalase was worked out in 1969. The latest activity involving catalase was in 1981 when its 3D structure of the enzyme was revealed.
The function of catalase is to catalyze the decomposition of hydrogen peroxide, a common antiseptic, bleaching agent, but is a harmful by product of many normal metabolic processes. The products of the reaction being water and oxygen.
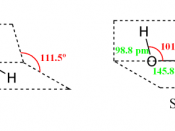
2H2O2 2H2 + O2ÃÂHo of âÂÂ98.2 kJÃÂmolâÂÂ1ÃÂGo of âÂÂ119.2 kJÃÂmolâÂÂ1ÃÂS of 70.5
JÃÂmolâÂÂ1ÃÂKâÂÂ1.
Catalase is used by cells to rapidly decompose hydrogen peroxide, creating less reactive products. It has one of the highest turnover ratios of any enzyme; one molecule of catalase will convert millions of molecules of hydrogen peroxide per second. Catalase is a tetramer of four polypeptide chains, each including over 500 amino acids. The tetramer contains four porphyrin heme (iron) groups which permits the enzyme to react with hydrogen peroxide. All known animals use catalase in every organ, with an exceptionally high concentration existing in the liver. Within cells it is usually located in cellular organelle called the peroxisome. A major function of the peroxisome is breaking down fatty acids through a process called beta-oxidation, where fatty acids are broken down two carbons at a time and transformed to Acetyl-CoA. After which Acetyl-CoA is transferred back to the cytosol for usage. In animals beta-oxidation occurs in the mitochondria. In plant and yeast cells it occurs solely in the peroxisome. The peroxisome is also involved in symbiotic nitrogen fixation, which breaks apart the diatomic molecule nitrogen into two reactive nitrogen atoms.
Catalase can oxidise different toxins, such as formaldehyde, formic acid, phenols, and acids.
H2O2 + H2R 2H2O + RIe: H2O2 + H2CO 2H2O + COFormaldehyde (methanal)H2O2 + H2CO2 2H2O + CO2Formic acid (methanoic acid)In this any heavy metal ion (copper, copper (II) sulphate) will perform as a non-competitive inhibitor on catalase. The complete mechanism for Catalase is not yet known, but it most likely occurs in two stages:H2O2 + Fe(III)-E H2O + O=Fe(IV)-E(,+)H2O2 + O=Fe(IV)-E(,+) H2O + Fe(III)-E + O2Fe( )-E represents iron (centre of heme group) attached to the enzyme.
Fe(IV)-E(.+) represents a mesomeric form of Fe(V)-E where iron is not completely oxidized to (V) but it receives some ÃÂsupporting electronsÃÂ from a heme ligand, heme being illustrated as (.+).
When hydrogen peroxide enters the active site it interacts with the amino acids Asn147 (asparagines at position 147) and His74 (Histidine).This causes a proton to transfer between the oxygen atom, which coordinates itself freeing the formed water molecule and Fe(IV)=O. After this Fe(IV)=O reacts with the second hydrogen peroxide molecule to redevelop Fe(III)-E, producing water and oxygen.
The catalase test is one of the main three tests used by microbiologists to classify categories of bacteria. The presence of the catalase enzyme in the test is detected using hydrogen peroxide. In the test, if the bacteria being tested possess catalase when a small amount of isolated bacteria is added to hydrogen peroxide it bubbles (positive test). A positive test included bacteria labeled staphylococci and micrococci. With a negative test including streptococci and enterococci,Human applications of catalase include the food, textile, and aesthetics industries, as well as for contact lens hygiene. In the food industry it is used to remove hydrogen peroxide from milk previous to cheese production. Hydrogen peroxide is used in the productions of cheeses, such as swiss, to preserve natural milk enzymes, which are beneficial to the flavour of cheese.
Catalase is used to remove hydrogen peroxide from fabrics to ensure that the material is peroxide free. Within the textile industry hydrogen peroxide is used to bleach fabrics, catalase removes any traces of it before the fabric is dyed a multitude of different colours. Catalase in used in several mast treatments where estiticians combine the enzyme with hydrogen peroxide and place the combination on the face. The intent of this is to oxidize the cells in the upper layers of the epidermis. A few contact lenses cleaning products contain hydrogen peroxide to disinfect lenses. After, a solution which contains catalase is use to decompose hydrogen peroxide into water and oxygen, so the lenses can be safely worn. Catalase is also used to remove glucose form food systems, removing oxygen from beverages, in the production of fructose from starch, and production of gluconic acid.
Works Cited"Catalase: Facts, Discussion Form, and Encyclopedia Article." 2009. AbsoluteAstronomy.com. 16 Feb. 2009 .
"Catalase." MadSci FAQ: CATALASE. 1995-2006. MadSciNet. 28 Feb. 2009 .
Crook, James. "Catalase- An Extraordinary Enzyme." 5 July 2003. 16 Feb. 2009 .
Faraci, Frank M. "Hydrogen Peroxide: Watery Fuel for Change in Vascular Biology." Hydrogen Peroxide: Watery Fuel for Change in Vascular Biology-- Faraci 26 (9): 1931-- Arterio. 2006. American Heart Association, Inc. 28 Feb. 2009 .
Galembeck, Fernando. "The application of catalase for the elimination of hydrogen peroxide." V7n3a06.pdf. 2002. Anais da Academia Brasileira de CiÃÂências. 28 Feb. 2009 .
Marcey, David. "Catalase: H2O2 H2O2 Oxidoreductase." Catalase (hydrogen peroxide: hydrogen peroxide- oxidoreductase, EC 1.11.1.6). 2001. 16 Feb. 2009 .
Smith, S.E. "What is a Peroxisome." 2003. Conjecture corporation. 28 Feb. 2009 .